Number of CHOLBAM capsules needed to achieve the recommended dosage1
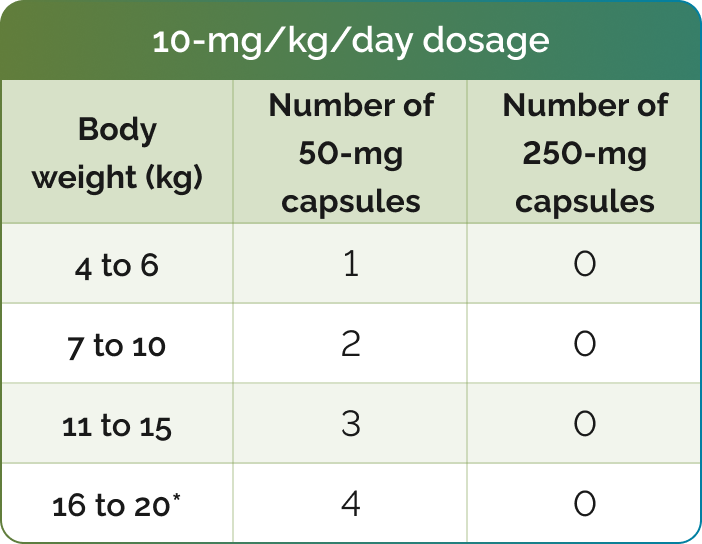
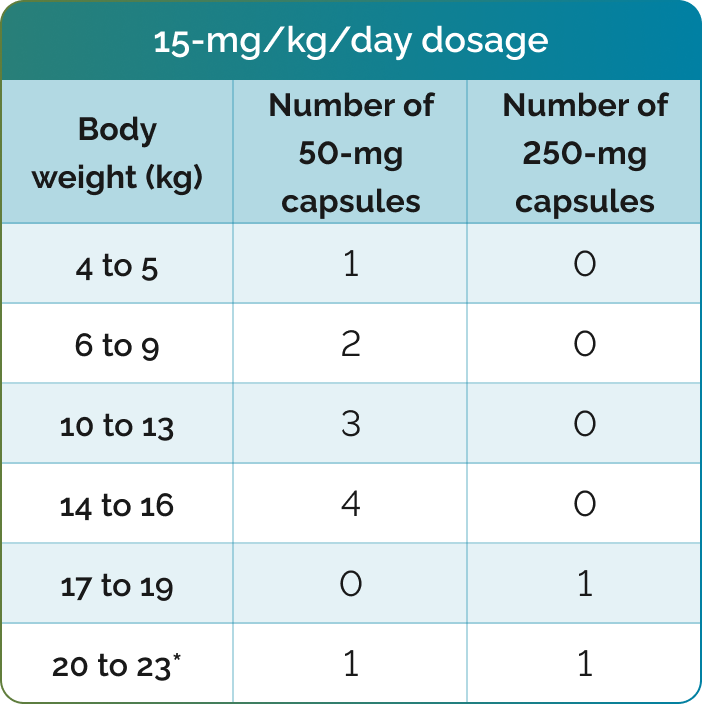
*For additional weights, please see dosing charts in the CHOLBAM Prescribing Information.
For patients with concomitant familial hypertriglyceridemia, the dosage is 11 mg/kg to 17 mg/kg once daily or in 2 divided doses and is adjusted based on clinical response.1
Please see the Prescribing Information for dose modification instructions for patients with newly diagnosed or a family history of familial hypertriglyceridemia, treatment monitoring guidelines, and administration instructions.
Administering CHOLBAM
CHOLBAM should be taken with food. For infants and children who cannot swallow capsules, CHOLBAM capsules can be opened and mixed with infant formula, expressed breast milk (for younger children), or soft food (for older children). Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin or aluminum-based antacid. Do not crush or chew the capsules.1
The recommended dosage of CHOLBAM is 10 mg/kg to 15 mg/kg taken once per day, or in 2 divided doses, in pediatric and adult patients. For patients starting therapy, consider twice-daily dosing as they adjust to having higher doses of cholic acid.

Administer the lowest dosage of CHOLBAM that effectively maintains liver function.1
Patients taking CHOLBAM should be carefully monitored1:
- Monitor aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), bilirubin, and international normalized ratio (INR) every month for the first 3 months, every 3 months for the next 9 months, every 6 months during the subsequent 3 years, and annually thereafter
- Monitor more frequently during periods of rapid growth, concomitant disease, and pregnancy
- The utility of bile acid measurements in monitoring the clinical course of patients and in decisions regarding dose adjustment has not been demonstrated
CHOLBAM has been used in real patients with PBD‑ZSD
INDICATIONS AND LIMITATIONS OF USE
CHOLBAM® (cholic acid) is a bile acid indicated for
•
Treatment of bile acid synthesis disorders due to single enzyme defects.
•
Adjunctive treatment of peroxisomal disorders, including Zellweger spectrum disorders, in patients who exhibit manifestations of liver disease, steatorrhea, or complications from decreased fat-soluble vitamin absorption.
LIMITATIONS OF USE
The safety and effectiveness of CHOLBAM on extrahepatic manifestations of bile acid synthesis disorders due to single enzyme defects or peroxisomal disorders, including Zellweger spectrum disorders, have not been established.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS – Exacerbation of liver impairment
•
Monitor liver function and discontinue CHOLBAM in patients who develop worsening of liver function while on treatment.
•
Concurrent elevations of serum gamma glutamyltransferase (GGT) and alanine aminotransferase (ALT) may indicate CHOLBAM overdose.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis.
ADVERSE REACTIONS
•
The most common adverse reactions (≥1%) are diarrhea, reflux esophagitis, malaise, jaundice, skin lesion, nausea, abdominal pain, intestinal polyp, urinary tract infection, and peripheral neuropathy.
DRUG INTERACTIONS
•
Inhibitors of Bile Acid Transporters: Avoid concomitant use of inhibitors of the bile salt efflux pump (BSEP) such as cyclosporine. Concomitant medications that inhibit canalicular membrane bile acid transporters such as the BSEP may exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. If concomitant use is deemed necessary, monitoring of serum transaminases and bilirubin is recommended.
•
Bile Acid Binding Resins: Bile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the efficacy of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin.
•
Aluminum-based Antacids: Aluminum-based antacids have been shown to adsorb bile acids in vitro and can reduce the bioavailability of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after an aluminum-based antacid.
PREGNANCY
No studies in pregnant women or animal reproduction studies have been conducted with CHOLBAM.
LACTATION
Endogenous cholic acid is present in human milk. Clinical lactation studies have not been conducted to assess the presence of CHOLBAM in human milk, the effects of CHOLBAM on the breastfed infant, or the effects of CHOLBAM on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CHOLBAM and any potential adverse effects on the breastfed infant from CHOLBAM or from the underlying maternal condition.
GERIATRIC USE
It is not known if elderly patients respond differently from younger patients.
HEPATIC IMPAIRMENT
•
Discontinue treatment with CHOLBAM if liver function does not improve within 3 months of the start of treatment.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
OVERDOSAGE
Concurrent elevations of serum GGT and serum ALT may indicate CHOLBAM overdose. In the event of overdose, the patient should be monitored and treated symptomatically. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
Please see full Prescribing Information for additional Important Safety Information.
Reference: 1. CHOLBAM® (cholic acid) capsules. Prescribing Information. Mirum Pharmaceuticals, Inc.



