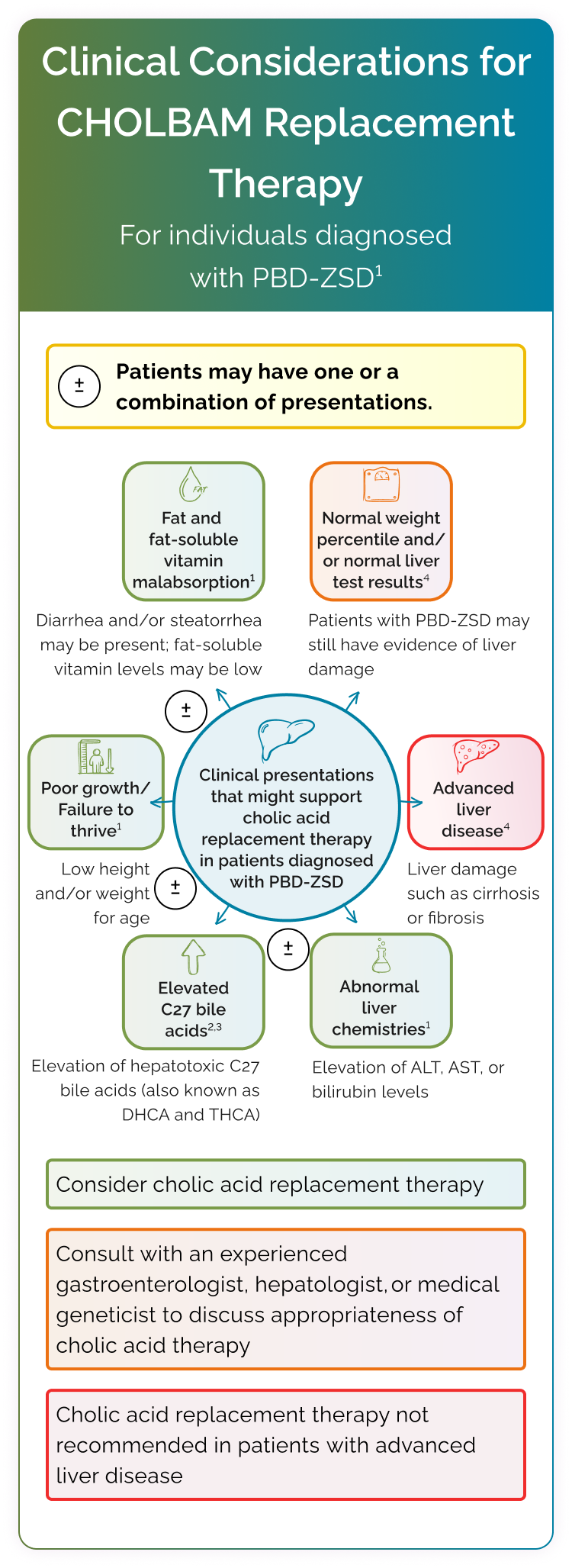
CHOLBAM works by increasing cholic acid levels1
By increasing cholic acid in the hepatocyte, CHOLBAM suppresses the production of hepatotoxic atypical bile acids.3
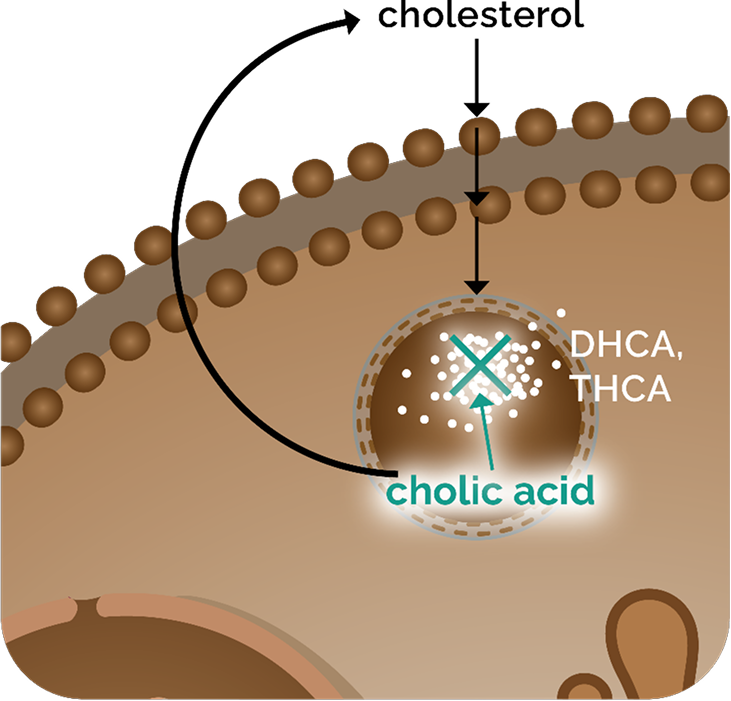
CHOLBAM in peroxisomal biogenesis disorder-Zellweger spectrum disorder (PBD‑ZSD) hepatocytes
In PBD-ZSD, defective peroxisomes disrupt bile acid synthesis, leading to3:
- An increase in dihydroxycholestanoic acid (DHCA) and trihydroxycholestanoic acid (THCA), 2 atypical bile acids shown to be hepatotoxic3
- A deficiency of the primary bile acids, including cholic acid3
Cholic acid deficiency disrupts bile acid homeostasis, impairs bile flow, and induces cholestasis.1
Orally administered CHOLBAM increases cholic acid levels to restore bile acid homeostasis1:
- While the exact mechanism of action of cholic acid has not been fully established, it is known that cholic acid acts as an endogenous ligand of the farnesoid X receptor, which regulates bile acid synthesis1
CHOLBAM may help improve bile flow and increase absorption of dietary fats and fat-soluble vitamins.3
CHOLBAM has been shown to improve liver function parameters and support weight gain in patients with PBD‑ZSD with liver involvement1
Results of 2 trials, including an 18-year, open-label trial and a 21-month extension, of patients with PBD‑ZSD (N=24)1†
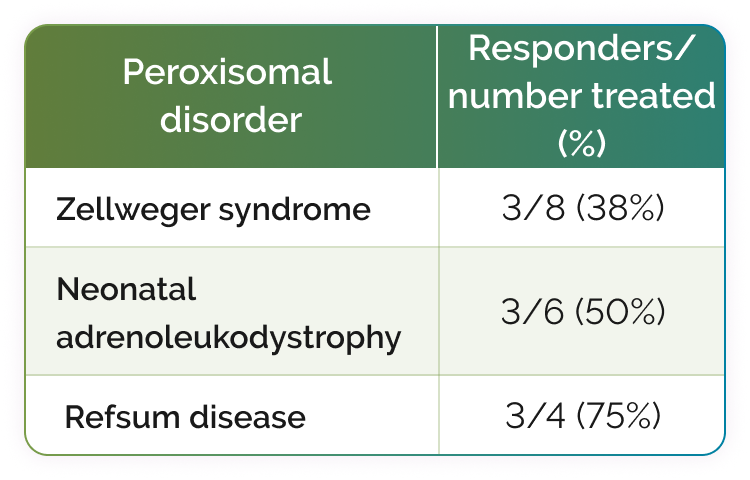
Two additional responders are not shown; 1 had generalized peroxisomal disorder and 1 had an unknown peroxisomal disorder.1
46% (11/24) of evaluable patients responded to CHOLBAM treatment.1‡

38% of the responders met the 2 clinical criteria plus ≥1 of the following laboratory criteria1:
- Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) values reduced to less than 50 U/L, or baseline levels reduced by 80%
- Total bilirubin values reduced to ≤1 mg/dL
- No evidence of cholestasis on liver biopsy was observed

63% of responders (7/11) showed improved weight gain1:
- Body weight increased by 10% or stabilized at >50th percentile
Response Criteria
Response to CHOLBAM treatment was assessed using the following criteria1:
- Laboratory criteria: (1) ALT or AST values reduced to <50 U/L, or baseline levels reduced by 80%; (2) total bilirubin values reduced to ≤1 mg/dL; and (3) no evidence of cholestasis on liver biopsy
- Clinical criteria: (1) body weight increased by 10% or stable at >50th percentile; (2) survival for >3 years on treatment or alive at the end of Trial 2
Clinical criteria: (1) body weight increased by 10% or stable at >50th percentile; (2) survival for >3 years on treatment or alive at the end of Trial 2
† Twenty-nine patients with PBD-ZSD were treated over an 18-year period in a nonrandomized, open-label, single-arm trial. Twelve patients were treated in an extension trial (2 new patients and 10 patients who rolled over from the original trial). Efficacy data are available for 21 months of treatment in the extension trial.1
‡ Sufficient data were available to assess baseline liver function and effects of CHOLBAM treatment in 23 patients in the original trial and in 1 new patient in Trial 2.1
CHOLBAM reduces toxic accumulation of atypical bile acids3
CHOLBAM provided a significant decrease in atypical bile acids in patients with PBD‑ZSD without advanced liver disease.3
In an open-label study of 19 patients living with PBD‑ZSD, CHOLBAM 15 mg/kg/day was administered to 15 patients without advanced liver disease and 4 patients with severe fibrosis or cirrhosis for a period of 9 months. The dosage was increased to 20 mg/kg/day if C27-bile acid intermediates DHCA and/or THCA were still detectable in plasma. In case of clinical side effects, particularly diarrhea, vomiting, or biochemical effects defined as a 2-fold increase in plasma transaminases or conjugated bilirubin, the dosage was reduced to 10 mg/kg/day.3§||
The effect of CHOLBAM on plasma DHCA and THCA in patients with PBD-ZSDs without advanced liver disease (n=15)3#
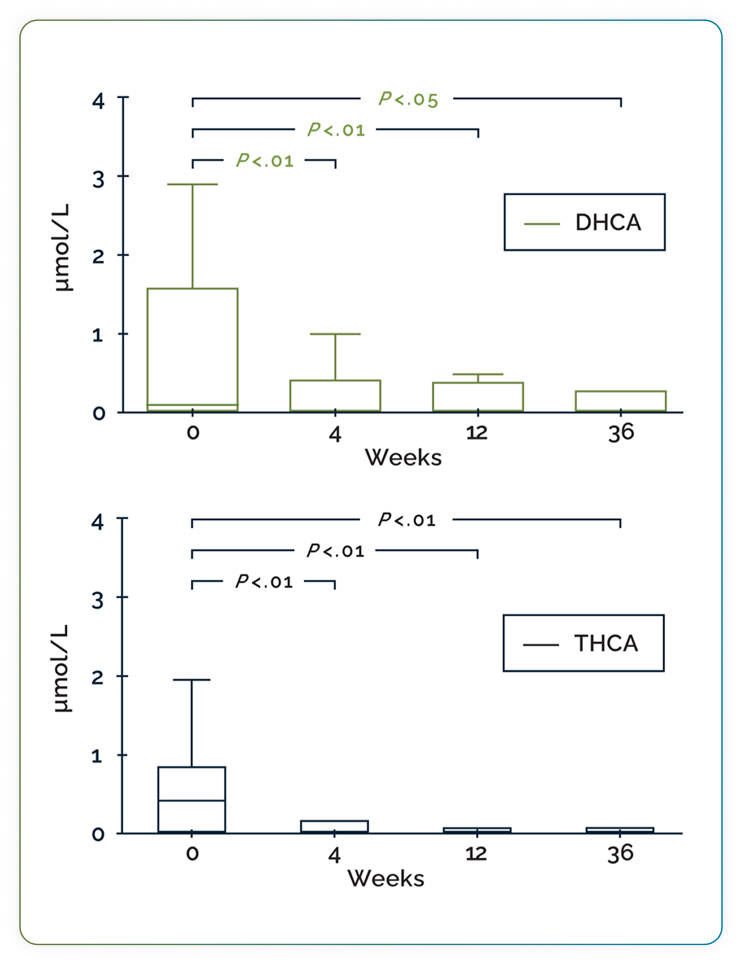
Box-and-whisker plots showing the effect of CHOLBAM therapy on atypical bile acid levels. Patients were followed for 2 to 2.5 years prior to the start of intervention with CHOLBAM. The reference range for THCA is <0.05–0.1 µmol/L; DHCA is undetectable in controls (<0.05 µmol/L). Statistical analyses were performed using a Wilcoxon matched-pairs signed-rank sum test. Adapted from Berendse K et al, J Inherit Metab Dis, 2016.
31% of patients (4/13) with elevated levels of DHCA/THCA at baseline had undetectable atypical bile acids after 9 months of CHOLBAM treatment3
Patients with advanced liver disease exhibited persistently elevated atypical bile acid levels during cholic acid treatment3
§ Liver stiffness was evaluated using ultrasound-based transient elastography (FibroScan®) and scored according to the Metavir fibrosis scoring system for chronic cholestatic liver disease. FibroScan® values ≥15.5 kPa were defined as severe fibrosis or cirrhosis.3
|| Please note that the recommended dosage is 10 to 15 mg/kg/day.1 Refer to full Prescribing Information for full dosage and administration information.1
# Outliers have been omitted from the graph.
Years of Clinical Data and Real-World Use
Backed by >18 years of safety data, CHOLBAM has a well-characterized safety profile in patients with PBD-ZSD. There were 12 adverse reactions reported across 9 patients in the clinical trials.1
CHOLBAM helps patients absorb what matters.
It has been FDA approved since 2015.1
Most common adverse reactions in Trials 1 and 21
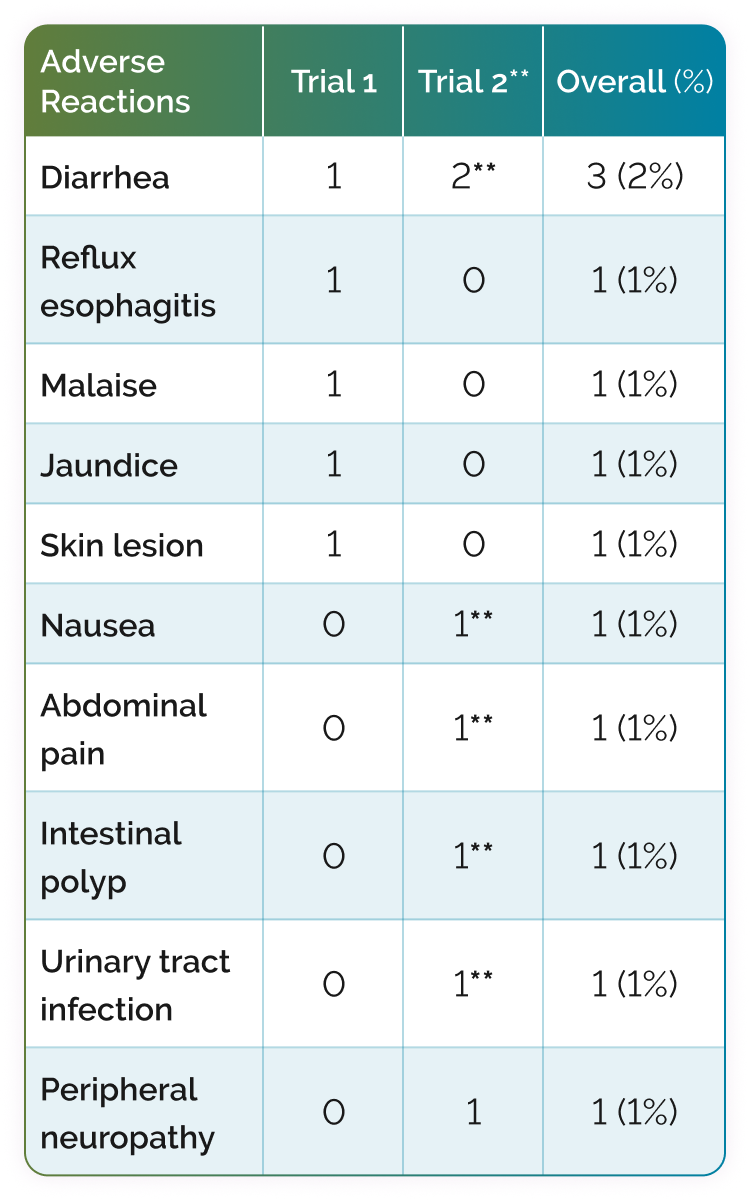
** Adverse reactions that occurred in new patients.

CHOLBAM is simple for patients to start

Help get your patients with PBD-ZSD started on CHOLBAM
INDICATIONS AND LIMITATIONS OF USE
CHOLBAM® (cholic acid) is a bile acid indicated for
•
Treatment of bile acid synthesis disorders due to single enzyme defects.
•
Adjunctive treatment of peroxisomal disorders, including Zellweger spectrum disorders, in patients who exhibit manifestations of liver disease, steatorrhea, or complications from decreased fat-soluble vitamin absorption.
LIMITATIONS OF USE
The safety and effectiveness of CHOLBAM on extrahepatic manifestations of bile acid synthesis disorders due to single enzyme defects or peroxisomal disorders, including Zellweger spectrum disorders, have not been established.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS – Exacerbation of liver impairment
•
Monitor liver function and discontinue CHOLBAM in patients who develop worsening of liver function while on treatment.
•
Concurrent elevations of serum gamma glutamyltransferase (GGT) and alanine aminotransferase (ALT) may indicate CHOLBAM overdose.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis.
ADVERSE REACTIONS
•
The most common adverse reactions (≥1%) are diarrhea, reflux esophagitis, malaise, jaundice, skin lesion, nausea, abdominal pain, intestinal polyp, urinary tract infection, and peripheral neuropathy.
DRUG INTERACTIONS
•
Inhibitors of Bile Acid Transporters: Avoid concomitant use of inhibitors of the bile salt efflux pump (BSEP) such as cyclosporine. Concomitant medications that inhibit canalicular membrane bile acid transporters such as the BSEP may exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. If concomitant use is deemed necessary, monitoring of serum transaminases and bilirubin is recommended.
•
Bile Acid Binding Resins: Bile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the efficacy of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin.
•
Aluminum-based Antacids: Aluminum-based antacids have been shown to adsorb bile acids in vitro and can reduce the bioavailability of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after an aluminum-based antacid.
PREGNANCY
No studies in pregnant women or animal reproduction studies have been conducted with CHOLBAM.
LACTATION
Endogenous cholic acid is present in human milk. Clinical lactation studies have not been conducted to assess the presence of CHOLBAM in human milk, the effects of CHOLBAM on the breastfed infant, or the effects of CHOLBAM on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CHOLBAM and any potential adverse effects on the breastfed infant from CHOLBAM or from the underlying maternal condition.
GERIATRIC USE
It is not known if elderly patients respond differently from younger patients.
HEPATIC IMPAIRMENT
•
Discontinue treatment with CHOLBAM if liver function does not improve within 3 months of the start of treatment.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
OVERDOSAGE
Concurrent elevations of serum GGT and serum ALT may indicate CHOLBAM overdose. In the event of overdose, the patient should be monitored and treated symptomatically. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
Please see full Prescribing Information for additional Important Safety Information.
References: 1. CHOLBAM® (cholic acid) capsules. Prescribing Information. Mirum Pharmaceuticals, Inc. 2. Heubi JE, Setchell KDR, Bove KE. Long-term cholic acid therapy in Zellweger spectrum disorders. Case Rep Gastroenterol. 2018;12(2):360-372. doi:10.1159/000490095 3. Berendse K, Klouwer FCC, Koot BGP, et al. Cholic acid therapy in Zellweger spectrum disorders. J Inherit Metab Dis. 2016;39(6):859-868. doi:10.1007/s10545-016-9962-9





