Bile acid synthesis disorder (BASD) due to single enzyme defects involves congenital deficiencies in enzymes required for catalyzing key reactions in the synthesis of the 2 main bile acids—cholic acid (CA) and chenodeoxycholic acid (CDCA).1
Often present in the first few months of life, BASD can produce life-threatening, progressive cholestatic liver disease2,3
It is crucial to diagnose and treat BASD as early as possible1,3,4
BASD accounts for ~2% of cholestatic jaundice cases4-6
Enzyme defects in the bile acid synthesis pathway can lead to liver disease2
- Cholesterol is converted to bile acids in the liver by a series of enzymatic reactions1,4
- Enzyme defects may occur anywhere along the pathway and in several different subcellular compartments8
- An accumulation of hepatotoxic atypical bile acids occurs above the defect in the pathway7
- Bile acids regulate their own synthesis via the farnesoid X receptor (FXR)7
Symptoms of BASD or inherited liver disease can sometimes be subtle1
Patients with mild BASD may present with any or all of the following4,7,9,10:
- Cholestasis
- Fat malabsorption
- Steatorrhea
- Failure to thrive
- Chronic diarrhea
- Malnourishment
- Deficiencies in fat-soluble vitamins (A, D, E, and K)
- Normal or slightly elevated liver enzymes
- Normal serum bile acid levels
BASD due to single enzyme defects has distinct clinical features
Common clinical features that are predominantly present in neonates and infants with BASD may also be seen in milder forms in adolescents and adults.11
Clinical Features1,4,7,9
Cholestatic jaundice
Acholic stools
Hepatomegaly
Failure to thrive
Absence of pruritus
Lab Abnormalities4,12
Lab abnormalities
BASD
Other types of cholestasis
Direct bilirubin (mg/dL)
Elevated
Elevated
ALT/AST (U/L)
Elevated
Elevated
GGT (U/L)
≤150
Variable
Primary bile acids (serum) (µmol/mL)
Normal to low
Elevated
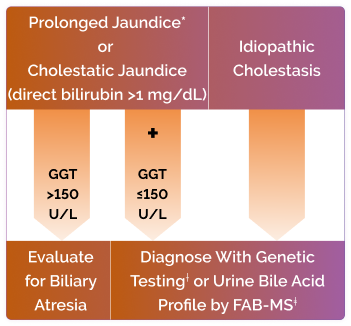
Early diagnosis of BASD is critical for optimal treatment outcomes1,3
Suspect BASD with the following hallmark features1,4,6,9,10,13,14:
* >2 weeks.
†eg, PreventionGenetics 77-gene cholestasis panel.
‡FAB-MS, Fast Atom Bombardment Mass Spectrometry at Cincinnati Children’s Hospital Center.
INDICATIONS AND LIMITATIONS OF USE
CHOLBAM® (cholic acid) is a bile acid indicated for
•
Treatment of bile acid synthesis disorders due to single enzyme defects.
•
Adjunctive treatment of peroxisomal disorders, including Zellweger spectrum disorders, in patients who exhibit manifestations of liver disease, steatorrhea, or complications from decreased fat-soluble vitamin absorption.
LIMITATIONS OF USE
The safety and effectiveness of CHOLBAM on extrahepatic manifestations of bile acid synthesis disorders due to single enzyme defects or peroxisomal disorders, including Zellweger spectrum disorders, have not been established.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS – Exacerbation of liver impairment
•
Monitor liver function and discontinue CHOLBAM in patients who develop worsening of liver function while on treatment.
•
Concurrent elevations of serum gamma glutamyltransferase (GGT) and alanine aminotransferase (ALT) may indicate CHOLBAM overdose.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis.
ADVERSE REACTIONS
•
The most common adverse reactions (≥1%) are diarrhea, reflux esophagitis, malaise, jaundice, skin lesion, nausea, abdominal pain, intestinal polyp, urinary tract infection, and peripheral neuropathy.
DRUG INTERACTIONS
•
Inhibitors of Bile Acid Transporters: Avoid concomitant use of inhibitors of the bile salt efflux pump (BSEP) such as cyclosporine. Concomitant medications that inhibit canalicular membrane bile acid transporters such as the BSEP may exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. If concomitant use is deemed necessary, monitoring of serum transaminases and bilirubin is recommended.
•
Bile Acid Binding Resins: Bile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the efficacy of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin.
•
Aluminum-based Antacids: Aluminum-based antacids have been shown to adsorb bile acids in vitro and can reduce the bioavailability of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after an aluminum-based antacid.
PREGNANCY
No studies in pregnant women or animal reproduction studies have been conducted with CHOLBAM.
LACTATION
Endogenous cholic acid is present in human milk. Clinical lactation studies have not been conducted to assess the presence of CHOLBAM in human milk, the effects of CHOLBAM on the breastfed infant, or the effects of CHOLBAM on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CHOLBAM and any potential adverse effects on the breastfed infant from CHOLBAM or from the underlying maternal condition.
GERIATRIC USE
It is not known if elderly patients respond differently from younger patients.
HEPATIC IMPAIRMENT
•
Discontinue treatment with CHOLBAM if liver function does not improve within 3 months of the start of treatment.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
OVERDOSAGE
Concurrent elevations of serum GGT and serum ALT may indicate CHOLBAM overdose. In the event of overdose, the patient should be monitored and treated symptomatically. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
Please see full Prescribing Information for additional Important Safety Information.
References: 1. Heubi JE, Bove KE, Setchell KDR. Oral cholic acid is efficacious and well tolerated in patients with bile acid synthesis and Zellweger spectrum disorders. J Pediatr Gastroenterol Nutr. 2017;65(3):321-326. doi:10.1097/MPG.0000000000001657 2. Bove KE, Heubi JE, Balistreri WF, Setchell KDR. Bile acid synthetic defects and liver disease: a comprehensive review. Pediatr Dev Pathol. 2004;7:315-334. doi:10.1007/s10024-002-1201-8 3. Clayton PT. Disorders of bile acid synthesis. J Inherit Metab Dis. 2011;34:593-604. doi:10.1007/s10545-010-9259-3604 4. Sundaram SS, Bove KE, Lovell MA, Sokol RJ. Mechanisms of disease: inborn errors of bile acid synthesis. Nat Clin Pract Gastroenterol Hepatol. 2008;5(8):456-468. doi:10.1038/ncpgasthep1179 5. Fischler B, Lamireau T. Cholestasis in the newborn and infant. Clin Res Hepatol Gastroenterol. 2014;38(3):263-267. doi:10.1016/j.clinre.2014.03.010 6. Lu F-T, Wu J-F, Hsu H-Y, et al. γ-Glutamyl transpeptidase level as a screening marker among diverse etiologies of infantile intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2014;59:695-701. doi:10.1097/MPG.0000000000000538 7. Gonzales E, Gerhardt MF, Fabre M, et al. Oral cholic acid for hereditary defects of primary bile acid synthesis: a safe and effective long-term therapy. Gastroenterology. 2009;137(4):1310-1320. doi:10.1053/j.gastro.2009.07.043 8. Ferdinandusse S, Houten SM. Peroxisomes and bile acid biosynthesis. Biochim Biophys Acta. 2006;1763(12):1427-1440. doi:10.1016/j.bbamcr.2006.09.001 9. Rinawi F, Iancu TC, Hartman C, et al. Fat malabsorption due to bile acid synthesis defect. Isr Med Assoc J. 2015;17(3):190-192. 10. Setchell KDR. Adolf Windaus Prize Lecture 2004. Defects in bile acid synthesis—specific and treatable causes of metabolic liver disease. In: Paumgartner G et al, eds. Bile Acid Biology and its Therapeutic Implications: Proceedings of the Falk Symposium 141 (XVIII International Bile Acid Meeting). 1st ed. Springer; 2005:3-16. 11. Heubi JE, Setchell KDR, Bove KE. Inborn errors of bile acid metabolism. Clin Liver Dis. 2018;22(4):671-687. doi:10.1016/j.cld.2018.06.006 12. Feldman AG, Sokol RJ. Neonatal cholestasis: updates on diagnostics, therapeutics, and prevention. NeoReviews. 2021;22(12):e819-e836. doi:10.1542/neo.22-12-e819 13. Moyer V, Freese DK, Whitington PF, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39(2):115-128. doi:10.1097/00005176-200408000-00001 14. Jancelewicz T, Barmherzig R, Chung CT-S, et al. A screening algorithm for the efficient exclusion of biliary atresia in infants with cholestatic jaundice. J Pediatr Surg. 2015;50(3):363-370. doi:10.1016/j.jpedsurg.2014.08.014